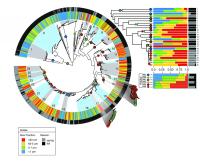
This figure shows the likely habitats of vibrio bacteria populations mapped onto a phylogenetic tree for all strains found in the samples taken from the Atlantic Ocean near Plum Island, Mass. Dot colors indicate the predicted habitat of the bacteria (red are believed to attach to zooplankton, yellow to large organic particles, green to small organic particles, and finally blue are free-floating). The outer ring indicates the microbe’s preference for warm weather (gray) or cold (black). The inner ring shows where the microbes were found (attached or free-floating). The 25 shaded bands within show the ecological populations based on habitat and genetic similarity, all of which originated from a common ancestor hundreds of millions of years ago. Populations 9 through 25 are all called V. splendidus, even though they have distinct habitat preferences and genetic lineage distributions. Two of those (15 and 17) appear to be in the process of radiating into a different habitat. Credit: Lawrence David and Dana Hunt, MIT Marine bacteria in the wild organize into professions or lifestyle groups that partition many resources rather than competing for them, so that microbes with one lifestyle, such as free-floating cells, flourish in proximity with closely related microbes that may spend life attached to zooplankton or algae.
This new information about microbial groups and the methodology behind it could change the way scientists approach the classification of microbes by making it possible to determine on a large scale, relatively speaking, the genetic basis for ecological niches. Microbes drive almost all chemical reactions in the ocean; it’s important to identify the specific professions held by different groups.
“This is the first method to accurately differentiate the ecological niche or profession among large groups of microbes in the ocean,” said Professor Martin Polz, a microbiologist in MIT’s Department of Civil and Environmental Engineering. He and colleague Professor Eric Alm, a computational biologist, published a paper describing their research in the May 23 issue of Science.
The nature of reproduction in microbes makes it impossible to define populations based on the ability of individuals within a species to share genes, as we do with larger animals. It’s only by determining bacteria’s ecological niche that scientists can classify them into populations. But microbes don’t live in natural population groups when cultured in a lab. So scientists must catch bacteria in the wild, then examine them genetically to determine their lifestyle.
“Most methods in use either over or underestimate greatly the number of microbial populations in a sample, leading either to a confusing array of populations, or a few large, but extremely diverse groups,” said Polz. “Eric’s method takes genetic information and groups the microbes into genetically distinct populations based on their preference for different habitats. Although this sounds like a simple problem, it is exceedingly difficult with microbes, because we have no species concept that would allow us to identify the genetic structure expected for populations. Microbial habitats differ on such small scales that they are invisible to us.”
Polz and former graduate student Dana Hunt, now a postdoctoral researcher at the University of Hawaii, created a large and accurate genetic data set by isolating and identifying over 1,000 strains of vibrio bacteria from a sample of eight liters of seawater gathered near Plum Island, Mass., in the spring and fall. To achieve accuracy in their identification of strains, they selected a gene whose molecular clock—the rate at which a gene accumulates random mutations over time—was well-suited to the task.
“The trick in many ways is choosing a gene that has a molecular clock that ticks at the right rate,” said Polz. “In particular, if it’s too slow, you might lump organisms into a single group that you would actually like to differentiate. We chose a gene that accumulates mutations fairly fast and thus allowed us to differentiate closely related groups of individuals and map the ecological data we collected onto their family tree.”
Alm and graduate student Lawrence David wrote an algorithm to make a conservative estimate of the minimum number of different habitats occupied by the vibrios (whether they live on small or large particles and thrive in the cool or warm months, etc.). They then combined information about habitat with phylogeny (the evolutionary history of groups of genes), and apportioned the original strains into 25 distinct populations and mapped their habitats back to a common ancestor, showing when and how each group diverged from the ancestral lifestyle.
“What is really new about our approach is that we were able to combine both molecular data (DNA sequences) with ecological data in a single mathematical framework,” said Alm. “This allowed us to solve the inverse problem of taking samples of organisms from different environments and figuring out their underlying habitats. In essence, we modeled the evolution of a microbe’s lifestyle over millions of years.”
One splendid example of the difficulty of applying the term “species” to a single-celled creature: 17 of those 25 populations are called V. splendidus, a name that was previously assigned to them based on classical taxonomic techniques. Alm and Polz can see now that V. splendidus has differentiated into several ecological populations.
Alm and Polz believe they caught at least one of those V. splendidus populations in the act of switching from one ecological niche (thriving on zooplankton) toward a new niche (attaching to small organic particles). Of course, this process takes millions of years, so the current population of scientists may never know for certain.
Source : Massachusetts Institute of Technology, Department of Civil and Environmental Engineering
 Print Article
Print Article Mail to a Friend
Mail to a Friend
