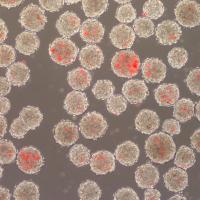
Fluorescence microscopy image overlaid with phase image to display incorporation of microspheres (red stain) in embryoid bodies (gray circles). New research shows that delivering molecules via biodegradable microspheres enhances the efficiency and purity of stem cell differentiation. Credit: Image courtesy of Todd McDevitt Embryonic stem cell therapies have been proposed for regenerative medicine and tissue replacement after injury or disease. However, the inability of stem cells to efficiently develop into the desired specific cell type – such as muscle, skin, blood vessels, bone or neurons – now limits the potential clinical utility of this therapy.
New research shows that delivering molecules within aggregates of embryonic stem cells via biodegradable microspheres enhances the efficiency and purity of differentiation, which is the process the cells undergo to become more specialized. Details of the microsphere-mediated delivery method, which is funded by the National Science Foundation, were presented on April 9 at the 235th American Chemical Society national meeting.
“Directing embryonic stem cells to efficiently differentiate into a specific cell type has been challenging to this point,” said Todd McDevitt, an assistant professor in the Wallace H. Coulter Department of Biomedical Engineering at Georgia Tech and Emory University. “In my lab, we’re trying to better define and then control the environmental cues that regulate the fate and function of the stem cells.”
Because physical interactions between stem cells is critical during normal embryonic development, most laboratory growth methods allow the cells to aggregate in three-dimensional clumps called “embryoid bodies” in order to differentiate. After individual cells aggregate together, hollow internal structures begin to develop and the aggregate becomes larger and more complex over time.
“Many researchers add soluble factors to the culture dish medium to direct differentiation, but this does not accurately mimic the time and location of signaling events present in normal development, and may contribute to heterogeneous differentiation,” said McDevitt. “Our method focuses on incorporating the differentiation factors directly into the cell aggregates in order to have a more controlled mechanism of presentation.”
The research team – which also includes graduate students Richard Carpenedo and Andrés Bratt-Leal and undergraduate students Ross Marklein and Scott Seaman – fabricated biodegradable polymer microspheres that could contain growth factors, proteins or other small molecules.
McDevitt’s team tested the impact of the poly(lactic-co-glycolic acid) (PLGA) microspheres on embryonic stem cell differentiation under different conditions by varying the microsphere-to-cell ratio and speed at which the aggregate cells were mixed with the microspheres. They also included a fluorescent dye in the microspheres so the degree of incorporation of the microspheres within the embryoid bodies could be assessed using fluorescent microscopy and spectroscopy.
The results revealed that the microspheres were incorporated into embryoid bodies under a variety of mixing conditions, but that slower rotary speeds and higher microsphere-to-cell ratios resulted in a greater degree of incorporation.
Next, the researchers compared differentiation of untreated cells, cells mixed with empty microspheres, cells mixed with retinoic acid-loaded microspheres, and cells treated with soluble retinoic acid. Retinoic acid was chosen initially because it is a potent inducer of embryonic stem cell differentiation.
After ten days, approximately 90 percent of the embryoid bodies mixed with retinoic acid-loaded microspheres began to display the hollow structure signifying differentiation, compared to 6 percent of the untreated bodies, 10 percent of the bodies coated with soluble retinoic acid, and 30 percent of the bodies mixed with empty microspheres. In addition, thirty percent of the embryoid bodies mixed with retinoic acid-loaded microspheres were completely hollow in the center, compared to nearly zero percent for the other groups.
“These results suggest that if you can control the signaling by presenting molecules locally on the inside of the embryoid body from biodegradable microspheres, you can effectively change the course and synchrony of differentiation,” said McDevitt.
To examine the cells in more detail, McDevitt teamed with Georgia Tech School of Biology chair John McDonald and research scientist Nathan Bowen to conduct microarray gene expression studies to determine cell phenotype.
The results revealed enhanced expression of fibroblast growth factor 5 (FGF-5) – a marker for primitive ectoderm – in the embryoid bodies mixed with retinoic acid-loaded microspheres compared to the other treatment groups after 10 days. The researchers also confirmed increased or inhibited expression of many additional markers.
“The importance of these findings is that we’ve shown that biomaterial-based approaches to regulate stem cell microenvironments can significantly improve differentiation methods,” said McDevitt. “Our ultimate goal is to improve the efficiency of this differentiation process into specific cell types for cell replacement therapies.”
Source : Georgia Institute of Technology Research News
 Print Article
Print Article Mail to a Friend
Mail to a Friend
