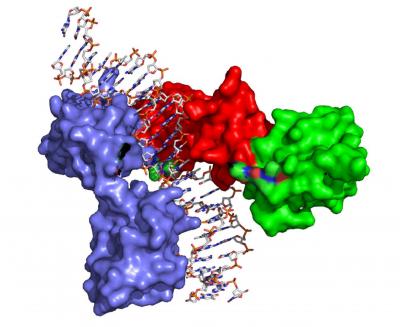
Space-filling model of Reb1 bound to DNA. In a study published on 28 March 2016 in the Proceedings of the National Academy of Sciences, researchers at the Medical University of South Carolina (MUSC) and Virginia Commonwealth University have resolved the first protein structure in a family of proteins called transcription terminators. The crystal structure of the protein, called Reb1, provides insight into aging and cancer, according to Deepak Bastia, Ph.D., Endowed Chair for Biomedical Research in the MUSC Department of Biochemistry and Molecular Biology and co-senior author of the study.
During transcription, large molecular machines read genes by traveling along double-stranded DNA. This machinery simultaneously reads out the gene code in continually lengthening chains of single-stranded RNA. The RNA code is then used to assemble proteins that cells use for growth and division. At certain times during the life of a cell, transcription must be stopped-in order to conserve cellular energy or prevent uncontrolled growth, for example. At other times, cells may be preparing to divide, during which period trouble can arise.
Before a cell can divide, the DNA must be exactly replicated for use in the new cell. During part of this process, two types of machinery are now moving along the DNA strand-transcriptional machinery and replication machinery. In regions where the two machines are moving in opposite directions, collisions can occur and DNA broken, causing mutations. Harmful gene mutations can be passed into the new cell. That's where Reb1 comes in.
"One way to prevent genome instability is to prevent replication from colliding with transcription," says Bastia. "That's what these terminator proteins do."
Bastia's group knew that there are specific sites on the DNA strand called terminator regions to which Reb1 binds itself. Reb1 was thought of as a simple physical barrier that sits on DNA and blocks both the transcriptional and replication machinery from moving further along the DNA strand and colliding with each other. Then Bastia's group did an experiment to cut the transcription terminator region (tail) off of Reb1. Intriguingly, Reb1 was no longer able to halt the transcription machinery without its tail but was still able to bind to DNA. Therefore, the simple roadblock theory couldn't be correct.
The insight came when they solved the crystal structure-a laborious process during which Carlos R. Escalante, Ph.D., Bastia's co-senior author from Virginia Commonwealth University, made monthly drives transporting freshly made crystals from MUSC to the X-ray crystallography facility at Brookhaven National Laboratory in New York. The crystal structure showed that, when bound to DNA, the transcription terminator tail of Reb1 can interact with a specific part of the transcriptional machinery, acting as a tether between the two.
The work illuminates Reb1 as a traffic signal for coordinating transcription and gene replication, rather than as a simple roadblock as previously thought.
Though the tether between Reb1 and the transcriptional machine is clear, the team is still not sure exactly how terminator proteins stop transcription, a question which drives their current work. And the connection between terminator proteins and colorectal cancer has been made, but work in other cancers and in aging has yet to be undertaken.
Still, Bastia suspects that this coordination prevents the type of gene errors that lead to many types of cellular aging and tumor growth, both of which are processes that result from uncontrolled transcription and replication. The group is currently researching another type of terminator protein, work which Bastia hopes will lend further knowledge to the diseases of aging.
Source: Medical University of South Carolina
 Print Article
Print Article Mail to a Friend
Mail to a Friend
