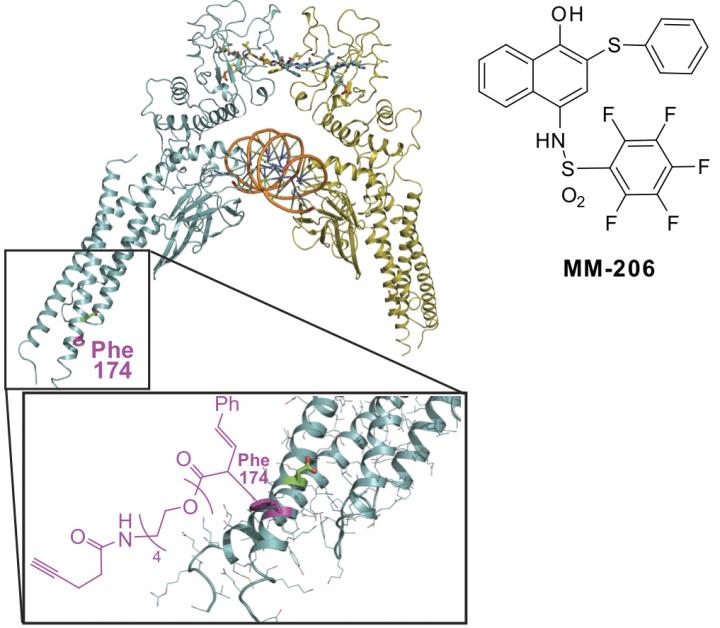
An illustration shows a molecule, MM-206, synthesized at Rice University and where it binds to a site on the coiled coil of a STAT3 protein. A protein domain once considered of little importance may be key to helping patients who are fighting acute myeloid leukemia (AML) avoid a relapse.
Researchers at Rice University, working with colleagues at Baylor College of Medicine and the University of Texas MD Anderson Cancer Center, have made a small molecule that could deliver a one-two punch to proteins that resist chemotherapy in patients with AML.
The protein, called STAT3, interferes with chemotherapy by halting the death of cancerous cells and allowing them to proliferate. The molecule discovered at Rice locates and then attacks a previously unknown binding site on STAT3, disrupting its disease-promoting effects.
The new work led by Rice chemist Zachary Ball, Baylor pediatrician Michele Redell and MD Anderson oncologist David Tweardy appears this week in the journal Angewandte Chemie.
The discovery and exploitation of this new drug target was made possible by an earlier discovery by Ball's lab. That finding enabled researchers to identify, on a molecular level, the target of action for drug molecules by using rhodium-based inorganic complexes that recognize specific folds in a protein chain and catalyze minute changes in those sequences, creating a "tag" for later analysis.
The STAT3 protein - it stands for "signal transducer and activator of transcription 3" - is a suspected factor in the relapse of nearly 40 percent of children with AML. The new proximity-driven rhodium(II) catalyst known as MM-206 finds and modifies an inhibitor-binding site on the protein's coiled coil -- literally protein coils coiled around each other -- and delivers the inhibitor, naphthalene sulfonamide, to the modified site.
"This is the confluence of two ideas we've been working on around what you can do with conjugates linked to rhodium," Ball said.
"We know that increased activity of STAT3 in AML and other cancers helps the cancer cells survive chemotherapy, so any new strategy we can develop to stop that process could mean real benefit for our patients," said Redell, who is also part of the leukemia and lymphoma teams at Texas Children's Hospital.
Ball said STAT3 has been a target for scientists trying to shut down cancer cells. "STAT fits in the broad category of what are called 'undruggable protein-protein interactions.' There's a large surface area with weak interactions for which we have typically failed to find good drugs," he said.
Previous research revolved around only one region of STAT3, its SH2 domain, with limited success. "There's no evidence people have tried to go after the coiled coil as a drug target," Ball said, though he noted one paper suggested it might be worth a look. "But they didn't follow up on it.
"Our main advance, from a medicinal perspective, is that this compound also works in a mouse model," he said. "All the other compounds worked in cells, but in mice, they weren't potent enough or stable enough."
Follow-up studies should lead to improved versions of the complex, Ball said. "The discovery raises new questions about STAT3 biology and points the way to future anti-cancer approaches, including combination therapies of coiled-coil STAT3 inhibitors in tandem with other agents," he said.
Source: Rice University
 Print Article
Print Article Mail to a Friend
Mail to a Friend
