The HIV envelope protein has long been considered one of the most difficult targets in structural biology and of great value for medical science Collaborating scientists at The Scripps Research Institute (TSRI) and Weill Cornell Medical College have determined the first atomic-level structure of the tripartite HIV envelope protein—long considered one of the most difficult targets in structural biology and of great value for medical science.
The new findings provide the most detailed picture yet of the AIDS-causing virus’s complex envelope, including sites that future vaccines will try to mimic to elicit a protective immune response.
“Most of the prior structural studies of this envelope complex focused on individual subunits; but we’ve needed the structure of the full complex to properly define the sites of vulnerability that could be targeted, for example with a vaccine,” said Ian A. Wilson, the Hansen Professor of Structural Biology at TSRI, and a senior author of the new research with biologists Andrew Ward and Bridget Carragher of TSRI and John Moore of Weill Cornell.
The findings are published in two papers in Science Express, the early online edition of the journal Science, on October 31, 2013.
A Difficult Target
HIV, the human immunodeficiency virus, currently infects about 34 million people globally, 10 percent of whom are children, according to World Health Organization estimates. Although antiviral drugs are now used to manage many HIV infections, especially in developed countries, scientists have long sought a vaccine that can prevent new infections and perhaps ultimately eradicate the virus from the human population.
However, none of the HIV vaccines tested so far has come close to providing adequate protection. This failure is due largely to the challenges posed by HIV’s envelope protein, known to virologists as Env.
Env’s structure is so complex and delicate that scientists have had great difficulty obtaining the protein in a form that is suitable for the atomic-resolution imaging necessary to understand it.
“It tends to fall apart, for example, even when it’s on the surface of the virus, so to study it we have to engineer it to be more stable,” said Ward, who is an assistant professor in TSRI’s Department of Integrative Structural and Computational Biology.
Illuminating Infection
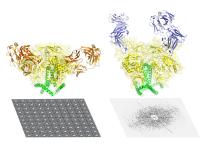
In the current work the Weill Cornell-TSRI team was able to engineer a version of the Env trimer (three-component structure) that has the stability and other properties needed for atomic-resolution imaging, yet retains virtually all the structures found on native Env.
Using cutting-edge imaging methods, electron microscopy (spearheaded by graduate student Dmitry Lyumkis) and X-ray crystallography (led by Jean-Philippe Julien, a senior research associate in the Wilson lab), the team was then able to look at the new Env trimer. The X-ray crystallography study was the first ever of an Env trimer, and both methods resolved the trimer structure to a finer level of detail than has been reported before.
The data illuminated the complex process by which the Env trimer assembles and later undergoes radical shape changes during infection and clarified how it compares to envelope proteins on other dangerous viruses, such as flu and Ebola.
“It has been a privilege for us to work with the Scripps team on this project,” said Moore on behalf of the Weill Cornell group. “Now we all need to harness this new knowledge to design and test next-generation trimers and see if we can induce the broadly active neutralizing antibodies an effective vaccine is going to need.”
Source : mikaono@scripps.edu
 Print Article
Print Article Mail to a Friend
Mail to a Friend
