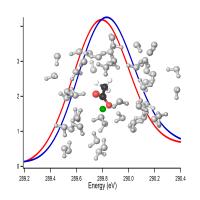
X-ray spectroscopy shows that a protein acetate group (molecule at center) prefers binding with sodium (blue curve) over potassium (red curve); the green sphere represents a cation, with surrounding water molecules in white. Credit: Lawrence Berkeley National Laboratory, figure by Janel Uejio In the late 19th century the Czech scientist Franz Hofmeister observed that some salts (ionic compounds) aided the solution of proteins in egg white, some caused the proteins to destabilize and precipitate, and others ranged in activity between these poles.
Hofmeister proceeded to rank "salt-out" (destabilizing) ions versus "salt-in" ions according to the magnitude of their effects. The resulting "Hofmeister series" governs the strengths of ions in inducing protein unfolding, bubble coalescence, and many other phenomena, and remains vital to protein chemistry and other biological and chemical studies to this day. But its mechanism has never been properly understood.
A team led by Richard Saykally of the Department of Energy's Lawrence Berkeley National Laboratory has now used Berkeley Lab's Advanced Light Source to study how biologically important, positively charged ions (cations) interact with negatively charged groups found in proteins (anions) to form salts. The team's results, which appear in Proceedings of the National Academy of Sciences, lend strong experimental support to a critical part of a proposed new explanation for Hofmeister effects, known as the Law of Matching Water Affinities.
The Law of Matching Water Affinities
"The Law of Matching Water Affinities, recently proposed by Kim Collins, says that the least soluble ion pairs are formed by ions that are closest to each other in their hydration energy -- that is, how strongly they hold onto water," says Saykally, who is a faculty scientist in Berkeley Lab's Chemical Sciences Division and a professor of chemistry at the University of California at Berkeley. "This is a classic example of an ion-specific effect: Hofmeister effects depend on the identity of ions rather than just on their concentration."
Hofmeister himself discovered that sodium salts-out egg white protein more efficiently than potassium, as does calcium. It's a difference with profound biological significance. "You don't want to precipitate salts inside the cells!" Saykally remarks. "That's part of why living organisms spend a lot of energy pumping calcium and sodium out of cells and pumping potassium in."
Computer simulations and quantum calculations of how sodium and potassium bind to proteins were performed by Luboš Vrbka and his colleagues in the Pavel Jungwirth research group, working in the Czech Republic in 2006. Their work indicated that the large difference between the binding efficiency of the two cations (which are otherwise similar in many ways) were consonant with the Law of Matching Water Affinities. In essence, Vrbka's simulations and calculations supported the Law's theoretical predictions.
Still needed was what Saykally calls "a new class of experimental support, stronger than previous experiments." His team, working with colleagues at beamline 8.0.1 of the Advanced Light Source, had developed just such an approach. Incorporating liquid microjet technology into the high-vacuum environment of a synchrotron x-ray experiment has allowed the group to perform near-edge x-ray absorption fine structure (NEXAFS) measurements on liquid samples that would otherwise be difficult or impractical to measure with synchrotron radiation.
Janel Uejio, a graduate student of Saykally's, recalls how she first became involved in the selective-binding investigation. She was working overnight on a different project at the 8.0.1 beamline when the phone rang at three o'clock in the morning.
"Rich had just read Vrbka's paper and had a brainstorm," says Uejio. "He wanted me to use liquid microjet technology to measure the selective binding of sodium and potassium to formate and acetate, two simple carboxylate groups characteristic of proteins. At that hour, all I had on hand were acetic acid and sodium chloride and potassium chloride" -- essentially, vinegar and table salt -- "but even so, the preliminary results were promising."
With liquid microjet technology, precisely mixed chemicals flow rapidly through a fused silica capillary shaped to a fine tip, a nozzle with an opening only a few micrometers (millionths of a meter) in diameter. The resulting liquid jet travels through a few centimeters of vacuum inside the beam chamber and is intersected by the synchrotron's x-ray beam, then collected by a cold skimmer and condensed out, to prevent any liquid molecules from contaminating the pristine vacuum of the synchrotron.
The great advantages of the system, says Saykally, are that in a vacuum the soft x-ray beam encounters only the liquid target -- there's no interference from air or windows or the like -- and that the rapid passage of the sample through the beam minimizes x-ray damage, which otherwise can be severe.
"In our NEXAFS experiment, the x-ray beam kicks the lowest-energy core electrons of the carbon on the carboxylate group up into the lowest empty antibonding molecular orbitals," Uejio explains. "The more tightly bound the cation is to the carboxylate, the more energy it takes to promote the electron. Therefore, the x-ray absorption spectra tell you about the relative binding energies of sodium, potassium, and lithium."
Kosmotropes versus chaotropes
The results confirmed Vrbka et al's models and further supported the Law of Matching Water Affinities -- thus lending weight to a growing trend in the interpretation of the venerable Hofmeister series. Saykally explains that the ions in the Hofmeister series are traditionally divided into kosmotropes, which bind strongly to water and supposedly structure it, and chaotropes, which bind only weakly to water and destructure it.
"There has been a widely held view that the Hofmeister series reflects changes in the bulk structuring of water -- that salting-out results when ions orient water molecules over a long range, reducing their density and allowing the protein to precipitate," he says. "But a more modern view is emerging, one exemplified by the Law of Matching Water Affinities, which suggests that Hofmeister effects are not due to such long-range perturbations of water but instead operate at a very short range, over a distance of only one or two molecules."
Of the dominant biological cations, for example, calcium and sodium are weak kosmotropes and potassium is a weak chaotrope. Says Saykally, "What matters is not whether individual cations are kosmotropes or chaotropes but how closely they are matched when they pair up with anions -- whether, in terms of hydration energy, they are two of a kind."
By using liquid microjet technology and x-ray absorption spectroscopy to characterize how sodium, potassium, and lithium interact with carboxylate groups, which are among the dominant anions in biological systems (and themselves kosmotropes), Saykally and his colleague have opened a new path toward probing selective interactions of ions with biological molecules in the aqueous environments typical of their natural surroundings. And they have gained new insight into the workings of the Hofmeister series, which has been called "as important to protein chemistry as Mendel was to genetics."
Source : DOE/Lawrence Berkeley National Laboratory
 Print Article
Print Article Mail to a Friend
Mail to a Friend
