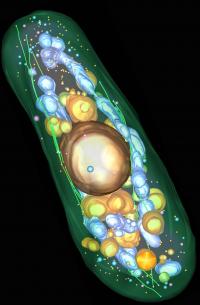
The electron tomogram of a complete yeast cell reveals the cellular architecture. It shows plasma membrane, microtubules and light vacuoles (green), nucleus, dark vacuoles and dark vesicles (gold), mitochondria and large dark vesicles (blue) and light vesicles (pink). Credit: Johanna Höög, EMBL Heidelberg Editor: A high resolution version of the picture is available!
Like our body every cell has a skeleton that provides it with a shape, confers rigidity and protects its fragile inner workings. The cytoskeleton is built of long protein filaments that assemble into networks whose overall architecture and fine detail can only be revealed with high resolution electron microscopy images. Researchers at the European Molecular Biology Laboratory (EMBL) and the University of Colorado have now obtained the first 3D visualization of a complete eukaryotic cell at a resolution high enough to resolve the cytoskeleton's precise architectural plan in fission yeast. The image of this unicellular organism will be published in this week's issue of the journal Developmental Cell and reveals remarkable insights into the fine structure of the cytoskeleton as well as its interactions with other parts of the cell.
A key component of the cytoskeleton are long, tube-like filaments called microtubules. They are dynamic structures built of constantly growing and shrinking rows of elementary proteins called tubulins. To increase their rigidity microtubules associate in bundles and interact with stabilizing proteins in complex networks, which are essential for many cellular processes such as polar growth.
"To really understand the architecture of the cytoskeleton you have to see the entire cell in three dimensions," says Claude Antony, whose team carried out the research at EMBL, "but at the same time you need a very good resolution to be able to investigate its structural details. It is impossible to obtain such detailed images of a eukaryotic cell with normal microscopes."
To bridge the gap between global overview and structural detail Antony's team collaborated with yeast and electron microscopy expert Richard McIntosh at the University of Colorado. Using a technique called electron tomography, Johanna Höög, PhD student in Antony's lab, took pictures of sequential sections of a yeast cell from many different angles through an electron microscope and combined these snapshots into a 3D reconstruction on the computer. A similar principle is used to generate brain scans.
For the first time they could see directly what previous studies in fission yeast only suggested. In times when a cell is not dividing a microtubule bundle consists of 4-5 individual filaments that are physically connected with each other via minute bridges likely formed by proteins. In the networks created through this crosslinking the orientation of microtubules is crucial. The filaments are polar structures, their two ends grow and shrink at different rates. The study created a precise map indicating the location of all growing and shrinking microtubule ends in the cell.
The images also shed light on other important functions of microtubules, revealing that the cytoskeleton determines the correct positioning of mitochondria, the energy-producing organelles, throughout the cell.
"Our 3D image of fission yeast can serve as a reference map of the cell for all biologists interested in its architecture," says Johanna Höög. "You can extract information about all sorts of cellular structures and processes from it or use it to place findings into the spatial context of the cell."
Yeast is one of the most commonly used model organisms in biology. It has many similarities with higher eukaryotes, including multicellular organisms. Many of the insights gained into its cellular organisation are likely to apply also to mammals. In mammalian nerve cells, for example, microtubule bundles similar to those observed in yeast are essential for the transmission of the signal from cell to cell.
Source : European Molecular Biology Laboratory
 Print Article
Print Article Mail to a Friend
Mail to a Friend
