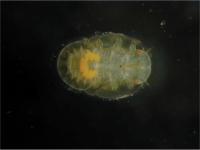
The bright yellow structure inside this newly hatched psyllid insect is the bacteriome, the special structure that houses endosymbiotic bacteria. The smallest collection of genes ever found for a cellular organism comes from tiny symbiotic bacteria that live inside special cells inside a small insect.
The bacteria Carsonella ruddii has the fewest genes of any cell. The bacteria's newly sequenced genome, the complete set of DNA for the organism, is only one-third the size of the previously reported "smallest" cellular genome.
"It's the smallest genome -- not by a bit but by a long way," said co-author Nancy A. Moran, UA Regents' Professor of ecology and evolutionary biology and a member of the National Academy of Sciences. "It's very surprising. It's unbelievable, really. We would not have predicted such a small size. It's believed that more genes are required for a cell to work."
Carsonella ruddii has only 159,662 base-pairs of DNA, which translates to only 182 protein-coding genes, reports a team of scientists from The University of Arizona in Tucson and from Japan.
The finding provides new insights into bacterial evolution, the scientists write in the Oct. 13 issue of the journal Science.
Atsushi Nakabachi, a postdoctoral research associate in UA's department of ecology and evolutionary biology and a visiting scientist at RIKEN in Wako, Japan, is the first author on the research report, "The 160-kilobase genome of the bacterial endosymbiont Carsonella." The research was conducted in senior author Masahira Hattori's laboratory in Japan and in Moran's lab at the UA. 
Hackberry petiole gall psyllid just after emerging from the gall on a hackberry. The leaf is yellow because it is autumn.
A complete list of authors is at the bottom of this release. The Ministry of Education, Culture, Sports, Science and Technology of Japan funded the work.
Many insects feed on plant sap, a nutrient-poor diet. To get a balanced diet, some sap-feeders rely on resident bacteria. The bacteria manufacture essential nutrients, particularly amino acids, and share the goodies with their hosts.
In many such associations, the bacteria live within the insect's cells and cannot survive on their own. Often the insect host cannot survive without its bacteria, known as endosymbionts.
The relationship between some insects and their endosymbionts is so close and so ancient that the insects house their resident bacteria in special cells called bacteriocytes within specialized structures called bacteriomes.
Studying the genomes of such endosymbionts can provide clues to how microorganisms' metabolic capabilities contribute to both their hosts and to biological communities.
An organism's genome, its complete complement of DNA, provides the operating instructions for everything the organism needs to do to survive and reproduce.
Endosymbiotic bacteria live in an extremely sheltered world and have a pared-down lifestyle, so they need a simpler set of instructions. Many of the metabolic pathways that free-living bacteria maintain have been lost after so many generations of living within insects.
Nakabachi and Hattori were interested in sequencing the genome of the bacteria Carsonella.
Moran had done some previous work on the Carsonella genome and found its DNA composition and evolution to be unusual. She suggested the team pursue the Carsonella that lived inside an Arizona psyllid insect called Pachypsylla venusta. The insect has only one species of endosymbiotic bacteria, which would simplify the genomic analysis.
The researchers collected Pachypsylla venusta psyllids from hackberry trees (Celtis reticulata) on the UA campus and around Tucson. The team extracted the Carsonella DNA and sequenced it.
Even though endosymbionts need fewer operating instructions to survive, the bacteria's itsy bitsy genome was a surprise.
"It lost genes that are considered absolutely necessary. Trying to explain it will probably help reveal how cells can work," said Moran, who is a member of UA's BIO5 Institute.
The scientists speculate that in the bacteria's evolutionary past, some of its genes were transferred into the insect's genome, allowing the insect to make some of the metabolites the bacteria needed. Once the insect shouldered those responsibilities and provided the bacteria with those metabolites, the bacteria lost those genes.
Animal and plant cells have specialized structures inside them called organelles that are derived from symbiotic bacteria that became incorporated into the cell over the course of evolution.
Carsonella's stripped-down genome may indicate that it is on its way to becoming an organelle, the researchers write in their article.
Source : University of Arizona
 Print Article
Print Article Mail to a Friend
Mail to a Friend
